New ” Ce ” Marking Regulation Entered into Force
CE Marking Regulation ( “Regulation” ) entered into force on the date it was published in the Official Gazette dated 27 May 2021 ; In this article, we will talk about the innovations and changes brought by the regulation.
- What is CE Mark? What is its shape?
First of all, the CE (“Conformité Européenne”) Mark is a health and safety mark created by the European Union in order to ensure the free movement of goods. If a product has this mark, it shows that it is healthy and safe for humans, animals and the environment.
In other words, this mark functions as a kind of passport for the products to be released for free circulation in EU member states.
- What has this Regulation brought different from the 2011 CE Marking Regulation? What is the Purpose and Scope of the Regulation?
First of all, with the CE Marking Regulation, it was aimed to determine which products should be CE marked and the procedures and principles regarding the use of this mark, and some obligations were introduced.
In fact, the procedures and principles in the use of CE Mark were determined by the CE Marking Regulation dated 2011. However, this regulation was issued based on Article 14 of the Law No. 4703 dated 2001. There were differences such as the fact that there was no clear CE marking in this article, and that only the procedures and principles of the notifications to be made to the EU were determined by the Undersecretariat of Foreign Trade. In addition, the Council of Ministers was responsible for the implementation of this regulation.
The Regulation, which came into force on May 27, 2021, which is the subject of this article , was issued based on Article 24 of the Law No. 7223 of 2020. Unlike Law No. 4703, Law No. 7223 clearly includes CE marking and it is stated that the President is authorized to determine the procedures and principles regarding this.
Therefore, when we compare the old-dated regulation with the Regulation that entered into force in 2021;
- Both have regulated the obligations regarding the manufacturer, importer and authorized representative; In addition, obligations for distributors are also included in the Regulation.
- While the Council of Ministers is responsible for the implementation of the 2011 regulation; The President is authorized to determine the procedures and principles for the implementation of the 2021 CE Mark Regulation.
In addition, with the entry into force of this Regulation, the “CE” Marking Regulation, which was put into effect with the Council of Ministers Decision dated 16/12/2011 and numbered 2011/2588, has been repealed.
- What Should Be Considered When Obtaining a CE Certificate?
In order for the manufacturer to obtain the CE Mark, it is necessary to;
- Determine the CE Directives/Regulations to which the product is subject,
- To make or have the test required for the product done,
- Product’s design, drawing, content, structure, test reports, label, production process, production and control instructions, final control information, product catalogue, user manual, etc. Preparation of a Technical File with its contents,
- Apply to the approved/authorized organization and to carry out CE Certificate Product Conformity assessment by them.
According to the technical file and the Product Evaluation Result, the CE Certificate is issued and the CE Mark is used.
- Where to Get CE Mark?
This certificate can be obtained from the certification bodies with the status of notified body on the website of the Ministry of Commerce or the Nando website of the European Commission.
If a notified body related to CE marking is assigned in the regulation, then the documents obtained from any other certification body that does not have the status of Notified Body are invalid.
- What are the Obligations of the Manufacturer?
It is obliged to;
- Put the CE Mark on the product,
- Issue the EU declaration of conformity or other documents showing conformity,
- Carry out or have conformity assessment procedures done,
- Edit the relevant technical file,
- Keep the documents for 10 years.
- What are the Obligations of the Authorized Representative?
It should be noted that the authorized representative refers to the natural or legal person residing in Turkey who is assigned in writing by the manufacturer to fulfill some of the manufacturer’s obligations on his behalf.
Authorized representative obligated to;
• Carry out or have the conformity assessment procedures carried out, provided that the manufacturer assigns it in writing,
• Issue the EU declaration of conformity or other documents showing conformity, unless otherwise stated in the relevant technical regulation,
• Put the CE mark on the product,
• Keep the documents for 10 years.
However, Even if the manufacturer has stated in writing, the authorized representative will not be able to issue the technical file.
- What are the Obligations of the Importer?
Importer obligated to;
• Confirm that the manufacturer fulfills its obligations set forth in the Regulation,
• Confirm that the product carries the CE Mark and that the necessary documents are available,
• Keep the documents for 10 years.
- What are the Distributor’s Obligations?
While no obligation regarding the distributor was foreseen in the former CE Mark Regulation, some obligations were also foreseen for the distributor with this Regulation. Here with the distributor, natural or legal person other than the importer and manufacturer, who makes the product available on the market.
Before making the product available on the market, it is obliged to verify that it carries the CE Mark and that there are conformity documents.
In addition, importers and distributors that place a product on the market under their own name or trademark or change a product made available on the market in a way that affects its compliance with the technical regulation or the general product safety legislation published by the Ministry of Commerce are also considered manufacturers within the scope of this Regulation.
- How is the CE Mark placed on the product?
With the 10th article of the Regulation, the general principles regarding the use of the CE Mark are determined as follows:
- It is the manufacturer’s responsibility to put and ensure that the CE Mark is placed on the product and to confirm whether the product complies with the technical regulations.
- CE mark will require a product to be placed in case of multiple technical regulations, CE marking on the product concerned technical regulations in presumption direction are performed by the entire industry is considered.
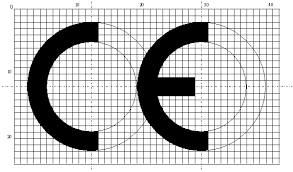
- It can be reduced and enlarged in accordance with the proportions of the CE Mark in the Regulation and the above image. Other than that, no changes can be made to the design.
- It is placed in a visible, legible, and indelible manner on the product or on the information plate, or in the packaging where this is not possible or the permanence of the product cannot be guaranteed due to the nature of the product, and in the documents accompanying the product stipulated by the relevant technical regulation.
- The CE Mark is placed only by the manufacturer or his authorized representative before the product is placed on the market.
• No other signs or descriptions that will mislead third parties about the meaning and form of the CE Mark can be placed on the product.
- What are the Regulations Regarding the EU Declaration of Conformity?
- If the EU declaration of conformity is stipulated in the relevant technical regulations, it is carried out by the manufacturer or his authorized representative to declare that the product complies with the technical regulations.
- Where the product is subject to more than one technical regulation requiring an EU declaration of conformity, the manufacturer demonstrates by issuing a single EU declaration of conformity that it fulfils all the rules of these technical regulations applicable to the product.
- If the relevant technical regulations stipulate that the product will be issued with a declaration of conformity other than the EU declaration of conformity, a declaration of conformity will be issued in accordance with the procedures and principles specified in the technical regulation.
